 SOPHIE Mulligan has fought off cancer three times – and is now planning her dream wedding after receiving revolutionary CAR-T cell therapy at The Christie NHS Foundation Trust.
SOPHIE Mulligan has fought off cancer three times – and is now planning her dream wedding after receiving revolutionary CAR-T cell therapy at The Christie NHS Foundation Trust.
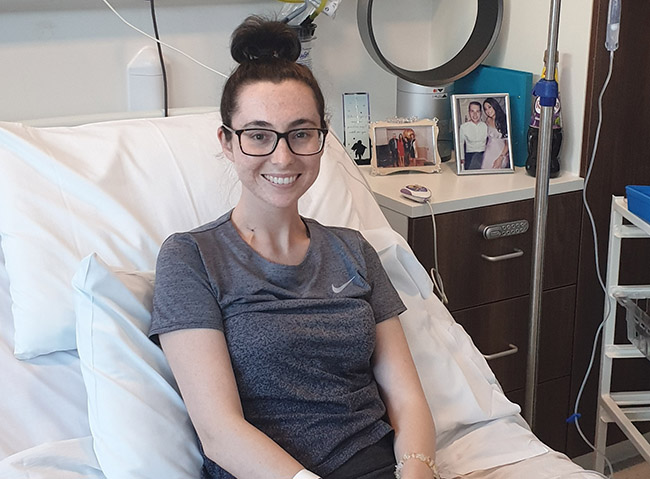
Sophie, 24, received the highly complex and innovative ‘personalised’ treatment at The Christie in May, having relapsed from acute lymphoblastic leukaemia (ALL) for the third time.
She can’t wait now to tie the knot with her fiancé Alex Maher, after receiving the state-of-the-art therapy which uses the body’s own cells to fight cancer.
CAR-T involves removing immune cells and modifying them in a laboratory so they can recognise cancer cells. It was approved by the NHS for appropriate and eligible patients last year.
Immune cells, called T-cells, are taken from a sample of the patient’s blood and reprogrammed in a lab to create ones that are genetically coded to recognise and destroy the patient’s cancer cells. This ’living therapy’ is then given to the patient.
Sophie was first diagnosed with leukaemia in 2015 while studying for a degree in Psychology at Liverpool John Moores University. Having felt run-down and lost weight she was diagnosed with the rare blood cancer. Despite repeated treatments, Sophie relapsed three times in five years but is now recovering thanks to CAR-T.
She received the treatment at The Christie in the midst of the COVID-19 pandemic, and recovered so well that she is now back at home and looking forward to planning her wedding.
 Sophie, who lives in Huyton, Liverpool, with mum Melanie, dad Ian, and younger sister Eve, said: “CAR-T cell therapy brought hope when I thought that there were no options left for me.
Sophie, who lives in Huyton, Liverpool, with mum Melanie, dad Ian, and younger sister Eve, said: “CAR-T cell therapy brought hope when I thought that there were no options left for me.
“Now, Alex and I can begin house hunting and planning our wedding, which will give our family and friends something to look forward to after a tough five years.”
First Sophie’s blood was taken and sent off to a laboratory in the United States of America, where her blood was ‘trained’ to fight the cancer cells. The CAR-T blood was then transported back to The Christie and Sophie was administered with it to treat her condition.
Sophie said: “To be told that my treatment was still going ahead during the Covid-19 pandemic brought very mixed emotions.
“I was glad to be dealing with the leukaemia, however I was afraid of possible exposure to the virus. However, the team at The Christie were phenomenal.
“They put my mind at ease as soon as I arrived onto the ward, and despite being unable to have visitors, the staff made sure that I never felt alone and that all of my needs were taken care of – they are all amazing.”
Whilst CAR-T is highly complex it is used to treat patients with advanced cancers but also comes with a number of risk factors.
Dr Amit Patel, Sophie’s physician at The Christie is a Consultant Haematology Oncologist and the Trust’s Lead for Cellular Therapies, said: “Successfully delivering such a complex treatment during the COVID-19 pandemic just shows how committed we are to ensuring our patients are given the best possible cancer care at the time it is needed here at The Christie.
“As with all our patients, we weighed up the risks and benefits associated with going ahead with the treatment during the COVID-19 pandemic as opposed to delaying or not offering Sophie CAR-T cell therapy, and together with Sophie we reached the decision it was the most appropriate course of action.
“We’re delighted to see Sophie respond so well to CAR-T cell therapy as she’s been through a lot over the past few years, including an intensive chemotherapy and an allogeneic donor stem cell (blood and marrow) transplant.”
“CAR-T cell therapy is transforming cancer treatment for some patients with blood cancers and this is a very exciting time to be involved with such an innovative treatment. It has the potential to change the way we treat cancer moving forward.”
- CAR-T therapy is designed to be a one-off treatment for people with advanced or progressing blood cancers, who have limited treatment options open to them.
- It is suitable for people with certain types of blood cancer who initially responded to treatment, but then relapsed (the cancer returned).
- It can also help those whose blood cancer is not responding to treatment (refractory or resistant disease).
- After treatment the patient is closely monitored with regular tests and check-ups for approximately two to three months to monitor their response, overall condition and watch for side effects.

